Schematic of seeding system used for acetone PLIF and PIV experiments.... | Download Scientific Diagram

Why does acetone remove the dark blue colour of the solution containing iodine, hcl, and starch, when thoroughly mixed? | Homework.Study.com

Dissociative Adsorption of Acetone on Platinum Single-Crystal Electrodes | The Journal of Physical Chemistry C

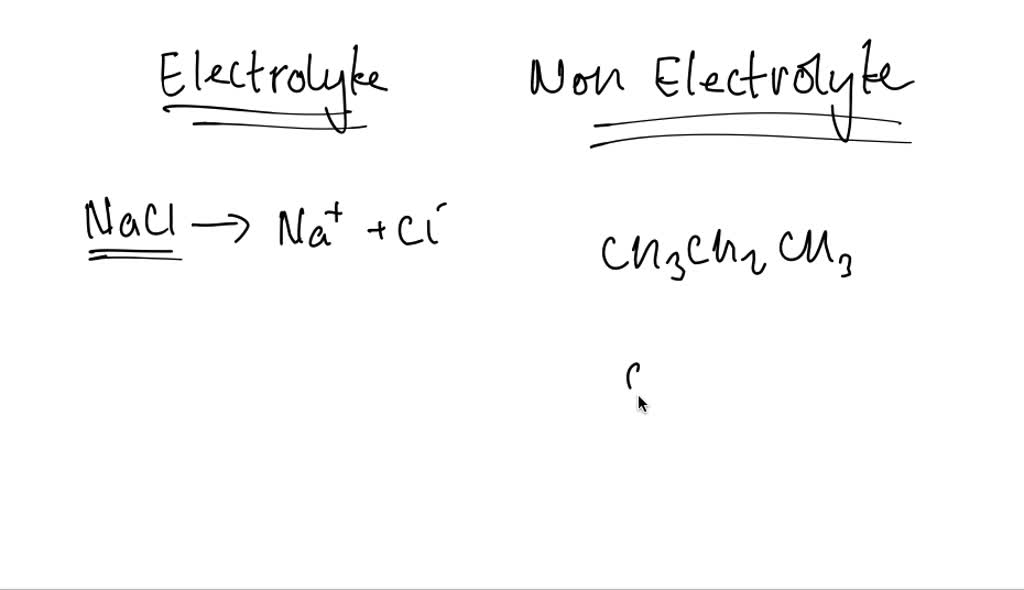
SOLVED:Predict whether each substance listed will conduct electricity, dissolve in water, and/ or conduct electricity once it has dissolved. Explain your thinking in each case. a. C3H6O(l) acetone b. Ti(s) titanium c.

22 Dilweniat de un moules and Minuous Aulous Memstulaus Argueous solution is me t A. Nen...dqueous solutum is wahich water is one in which watex sunt meta selvent. Example acclone, alchehal, borbon

Applied Sciences | Free Full-Text | Effect of Acetone Content on the Preparation Period and Curing/Pyrolysis Behavior of Liquid Polycarbosilane

Performance of a packed-bed anode bio-electrochemical reactor for power generation and for removal of gaseous acetone - ScienceDirect

similar ffer. jum eride Which statements are true regarding these two compounds? Check all that apply. - brainly.com

SOLVED: Dissolved in water. Which will NOT conduct electricity when dissolved in water? (A) HNO3 (nitric acid) (B) CH3COCH3 (acetone) (C) NaCl (sodium chloride) (D) KOH (potassium hydroxide) 31. Which is insoluble
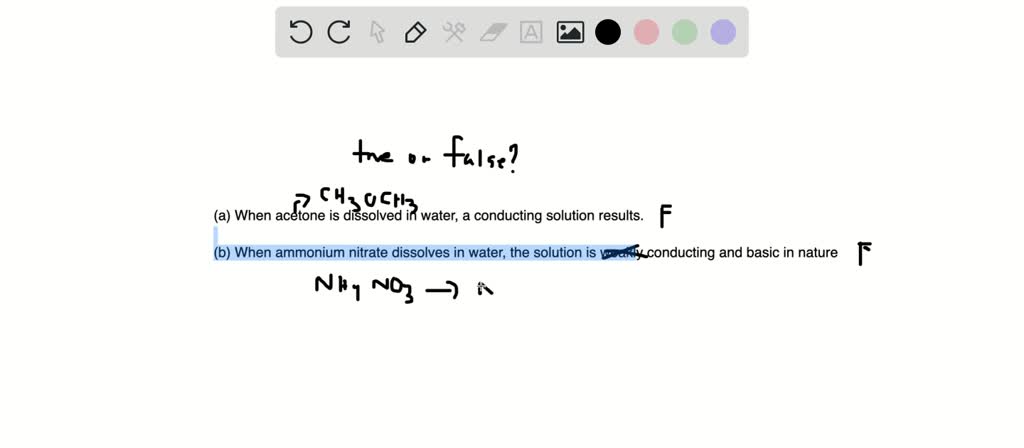
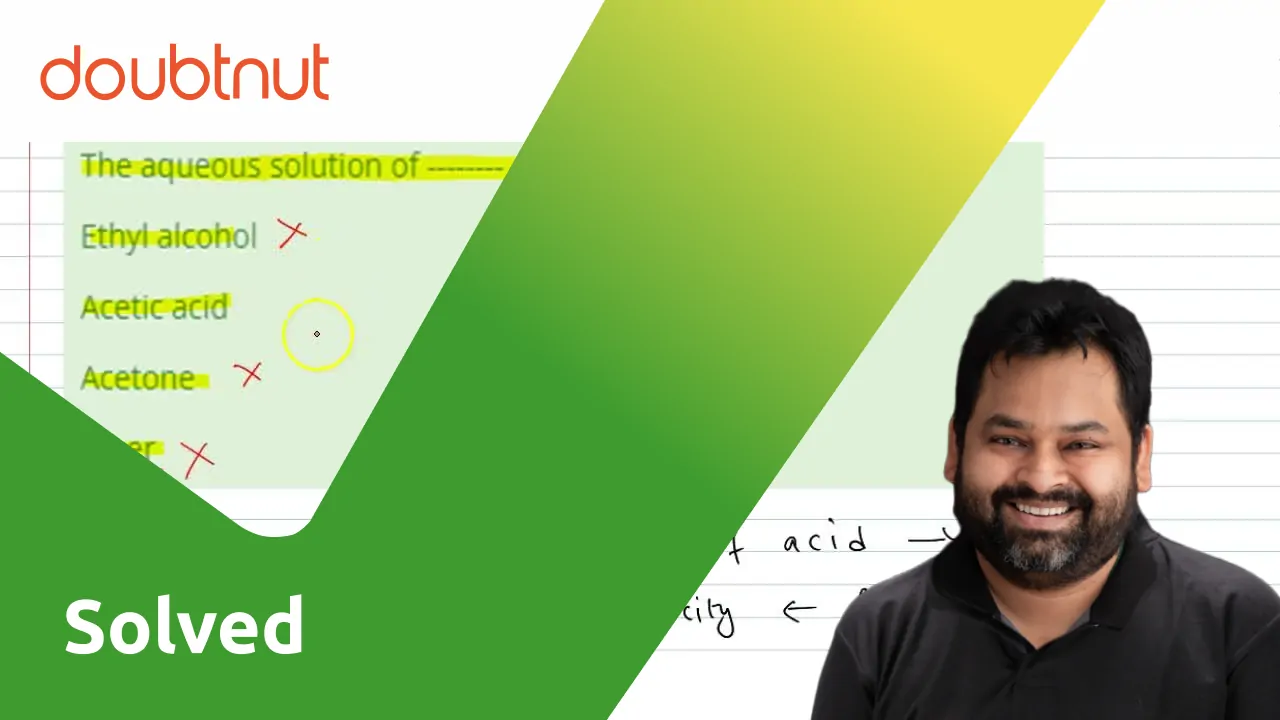
SOLVED:State whether each of the following statements is true or false. Justify your answer in each case. (a) When acetone, CH3 COCH3, is dissolved in water, a conducting solution results. (b) When
SOLVED: Which of the following when dissolved in water will conduct electricity? A. Methane - CH4 B. Carbon dioxide - CO2 C. Acetone - C3H6O D. Sodium Chloride - NaCl

Chapter 4 - General Chemistry - ▫ 6: multiply the empirical formula by this integer to determine - Studocu

SOLVED: Similar offer. Jump ride. Which statements are true regarding these two compounds? Check all that apply. Acetone is a covalent compound, while sodium chloride is an ionic compound. Ionic compounds conduct
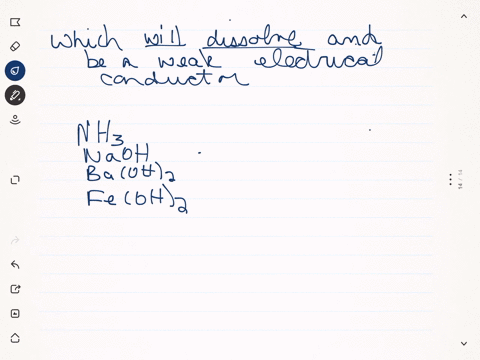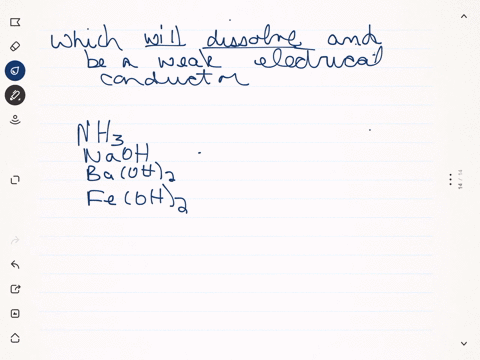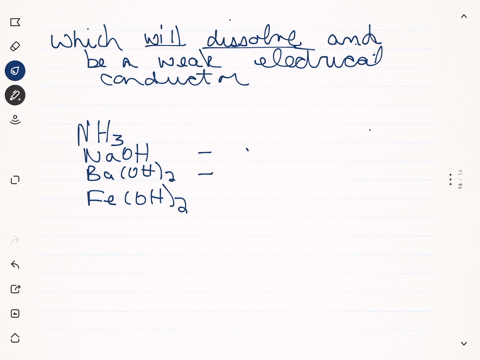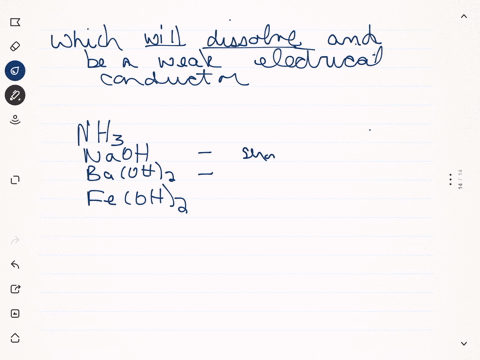
SOLVED:Predict whether each substance listed will conduct electricity, dissolve in water, and/ or conduct electricity once it has dissolved. Explain your thinking in each case. a. C3H6O(l) acetone b. Ti(s) titanium c.

Dissociative Adsorption of Acetone on Platinum Single-Crystal Electrodes | The Journal of Physical Chemistry C

similar ffer. jum eride Which statements are true regarding these two compounds? Check all that apply. - brainly.com

Performance of a packed-bed anode bio-electrochemical reactor for power generation and for removal of gaseous acetone - ScienceDirect
![Why is acetone used during the oxidation of 9-fluorenol with sodium hypochlorite solution and acetic acid? [{Image src='fluorenol3941635531123910328.jpg' alt='fluorenol' caption=''}] | Homework.Study.com Why is acetone used during the oxidation of 9-fluorenol with sodium hypochlorite solution and acetic acid? [{Image src='fluorenol3941635531123910328.jpg' alt='fluorenol' caption=''}] | Homework.Study.com](https://homework.study.com/cimages/multimages/16/fluorenol3941635531123910328.jpg)